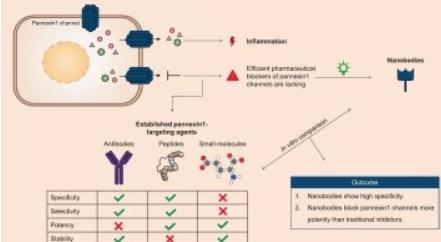
Pannexin1 channels mediate paracrine signaling and have recently emerged as key players in several inflammatory diseases. Although pannexin1 channel inhibition could represent a novel strategy for inflammatory disease treatment, therapeutic pannexin1 channel targeting is impeded by the lack of specific and potent inhibitors. To counteract this, we introduced nanobody-based inhibitors of pannexin1 channels. As previously demonstrated, the pannexin1-targeting nanobodies hold great promise as anti-inflammatory agents, yet this should be further tested. By using an in vitro approach, we continued the evaluation of the nanobodies by comparing their ability to bind and block pannexin1 channel activity with established pannexin1-binding compounds and pannexin1 channel inhibitors, respectively. Nanobodies directed to pannexin1 outperformed tested antibodies in terms of affinity towards human pannexin1 proteins, with their binding capacity being strongly linked to the extracellular loop regions of the pannexin1 protein, in an ELISA-based experimental setup. Moreover, the inhibitory effects of small molecule, peptide and nanobody-based inhibitors of pannexin1 channels were assessed by measurement of extracellular ATP release and intracellular YO-PRO1 uptake. Thus, carbenoxolone, probenecid, 10Panx1 and pannexin1-targeting nanobodies achieved blocking effects by reducing both ATP release and YO-PRO1 uptake. Collectively, the nanobodies show inhibitory effects by using smaller concentrations than traditional pannexin1 channel inhibitors. Therefore, nanobodies offer unique features for the development of pannexin1 channel inhibitors as novel anti-inflammatory therapeutics.
Link to Publisher’s page: A comparative in vitro analysis of pannexin1-targeting agents - ScienceDirect
