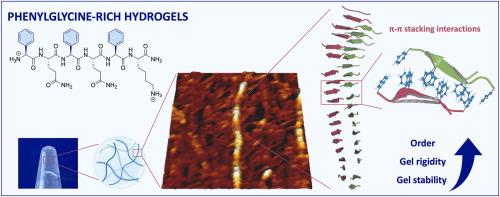
Peptide hydrogels are naturally inspired soft materials which, due to their biocompatibility, are potential candidates for controlled drug delivery matrices and wound healing applications. The properties of the gel materials are directly linked to the peptide sequence as minor alterations in the sequence are known to create substantial differences in the assembly mode. The majority of hydrogelators self-assemble by a combination of different non-covalent interactions, including hydrophobic effects, π-π stacking, ionic interactions and hydrogen bonding. Consequently, the impact and tunability of each separate interaction towards the self-assembly process is difficult to unravel. In this study, the role of aromatic interactions towards the self-assembly process of a hydrogelator is investigated by interchanging the more flexible phenylalanine amino acids with the more rigid phenylglycines in a short amphipathic hexamer peptide hydrogelator. This substitution resulted in four new effective hydrogelators that show different conformations around the aryl rings. The phenylglycine rich hydrogel SBL-HG-085 showed an increased gel strength by almost threefold, fast recovery after injection and improved stability under physiological conditions. The soft materials were further characterized at different levels and atomic models of their stacking modes were obtained by all-atom molecular dynamics simulations. A strong correlation has been achieved upon combining the theoretical and experimental results. Altogether, reducing the aromatic side chain flexibility stabilized the assemblies by modified π-π stacking interactions.
Link to Publisher’s page:
https://doi.org/10.1016/j.mtchem.2025.102593
