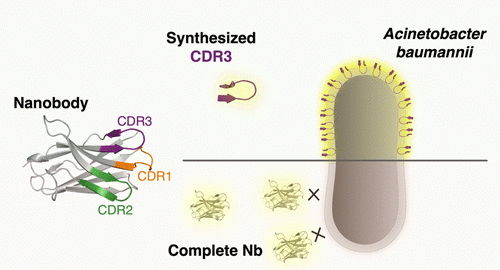
Membrane interaction constitutes to be an essential parameter in the mode of action of entities such as proteins, as well as cell-penetrating and antimicrobial peptides, resulting in noninvasive or lytic activities depending on the membrane compositions and interactions. Recently, a nanobody able to interact with the top priority, multidrug-resistant bacterial pathogen Acinetobacter baumannii was discovered, although
binding took place with fixed cells only. To potentially overcome this limitation, linear peptides corresponding to the complementarity-determining regions (CDR) were synthesized and fluorescently labeled. Microscopy data indicated clear membrane interactions of the CDR3 sequence with living A. baumannii cells, indicating both the importance of the CDR3 as part of the parent nanobody paratope and the improved binding ability and thus avoiding the need for permeabilization of the cells. In addition, cyclization of the peptide with an additionally introduced rigidifying 1,2,3-triazole bridge retains its binding ability while proteolytically protecting the peptide. Overall, this study resulted in the discovery of novel peptides binding a multidrug-resistant pathogen.
