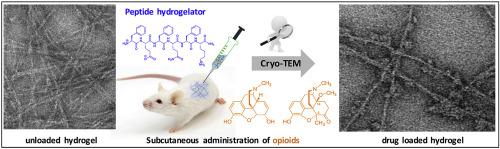
To overcome drawbacks related to repeated opioid administration during the treatment of chronic pain, several controlled-drug delivery systems of opioids have been designed. In order to address some of the limitations of the existing systems, injectable peptide-based hydrogels represent a promising alternative. This work reports on the design and synthesis of short amphipathic peptide-based hydrogels as controlled-drug delivery systems for opioids. Based on the lead sequence H-FEFQFK-NH2, a new set of peptide hydrogelators was designed including β-homo and d-amino acids, mainly aiming at enhancing proteolytic resistance of the peptides, and which hypothetically allows an extension of the drug release period. After self-assembly in aqueous media, the resulting hydrogels were characterized by dynamic rheometry, cryogenic transmission electronic microscopy and their cytotoxicity was assessed. The cryoTEM images of drug loaded hydrogels show the association of microcrystals of the loaded drug along the axes of the fibres, suggesting that the peptide fibres play a key-role as nucleating site for the drug crystals. Hydrogelators devoid of cytotoxicity were considered for further in vivoevaluation. Upon encapsulation of morphine and 14-methoxymetopon, two opioid analgesics, the applicability of the peptide hydrogels as controlled-drug delivery platforms was validated in vivo using the mouse tail-flick test. A sustained antinociceptive effect was observed after subcutaneous injection of the drug loaded gels and, in comparison with the lead sequence H-FEFQFK-NH2, novel sequences revealed extension of the in vivo antinociception up to 72–96 h post injection.
