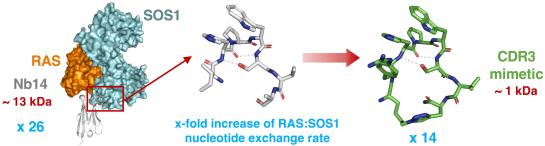
RAS proteins control various intracellular signaling networks. Mutations at specific locations were shown to stabilize their active guanosine triphosphate (GTP)-bound state, which is associated with the devel- opment of multiple cancers. An attractive approach to modulate RAS signaling is through its regulatory guanine nucleotide exchange factor (GEF) son of sevenless 1 (SOS1). With the recent discovery of Nano- body14 (Nb14), which potently enhances SOS1-cata- lyzed nucleotide exchange on RAS, we explored the feasibility of developing peptide mimetics by structurally mimicking the complementarity-determining region 3 (CDR3). Guided by a biochemical GEF assay and X-ray co-crystal structures, successive rounds of optimization and gradual conformational rigidification led to CDR3 mimetics showing half of the maximal activation poten- tial of Nb14 with an EC50 value of 29 μM. Altogether, this study demonstrated that peptides able to modulate a protein-protein interaction can be obtained by struc- tural mimicry of a Nb paratope.
