The Hennecke group is engaged in research in the area of organic and bioorganic chemistry. A key component of research focuses on organohalogen compounds. Since halogen substituents are common in natural products as well as active pharmaceutical ingredients and have a strong influence on physico-chemical properties, the group is developing synthetic methods for chemo-, regio- and stereoselective halogenation.
Enantioselective dichlorination of alkenes
The Hennecke group has recently developed a highly enantioselective dichlorination of unfunctionalized alkenes. The reaction is catalyzed by easily available cinchona alkaloid-based organocatalysts containing a phthalazine moiety. Carbocyclic as well as heterocyclic alkenes can be dichlorinated with high enantioselectivities:
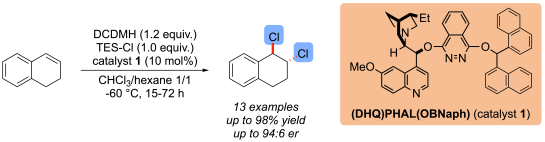
See: “Organocatalytic, Enantioselective Dichlorination of Unfunctionalized Alkenes”, V. Wedek, R. Van Lommel, C. G. Daniliuc, F. De Proft, U. Hennecke, Angew. Chem. Int. Ed. 2019, 58, 9239-9243.
Enantioselective halocyclisation
We have also contributed to the development of enantioselective halocyclization reactions such as halolactonisations and halocycloetherifications. For example, for halolactonisation, cinchona alkaloid-based catalysts can be used:
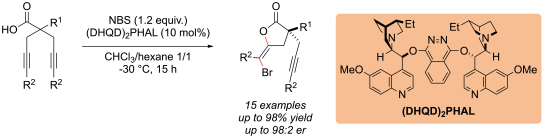
See: “Enantioselective, Desymmetrizing Bromolactonization of Alkynes”, M. Wilking, C. Mück-Lichtenfeld, C. G. Daniliuc, U. Hennecke, J. Am. Chem. Soc. 2013, 135, 8133-8136.
For a review on enantioselective halocyclisation, see:
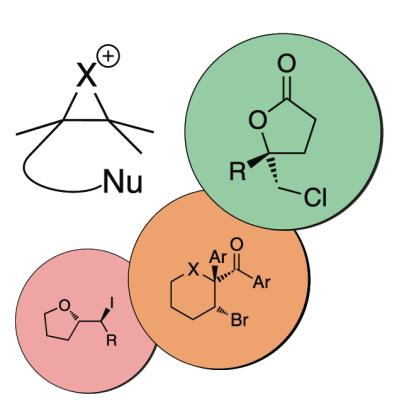
“New Catalytic Approaches towards the Enantioselective Halogenation of Alkenes”, U. Hennecke, Chem. Asian J. 2012, 7, 456-465.
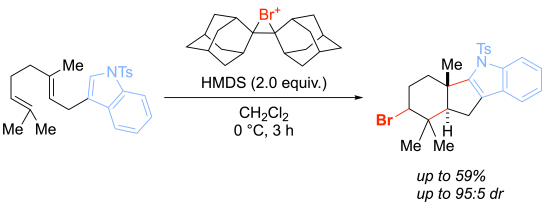
Halogen-induced polyene cyclisation
While the development of new enantioselective halogenation methods is a core area of research in the Hennecke group, we are also more broadly interested in other halogenation methodology and the synthesis of halogenated organic molecules with applications in biology and pharmaceutical sciences. For example, we have investigated halogen-induced polyene cyclizations on indole-derivatives. The resulting products are related to indole terpenoid natural products such as polyveoline, a natural product with activity against Plasmodium falciparum: