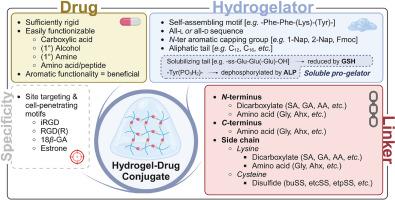
Efficacy of therapeutic drugs is oftentimes limited in time by their low metabolic stability and rapid clearance mechanisms. Peptide hydrogels have been used for the physical encapsulation of a vast number of drugs in the treatment of many different disease states, thereby effectively prolonging activity profiles of these drugs. However, consistent drug loading and release is generally lacking, while the delivery of hydrophobic drugs remains problematic. Many of these shortcomings can be addressed by covalently attaching the drug to the hydrogelator, thereby creating hydrogel-drug conjugates. Their easy synthesis and functionalization, alongside their biocompatibility, makes peptide hydrogels particularly fit for this purpose. Release can readily be tuned depending on the choice of linker connecting the drug to the hydrogelator, where it can be specifically directed towards the site of action, which limits undesired side effects. By attaching appropriate targeting motifs, additional site-selectivity can be obtained. This review provides an overview of the main principles applied in the development of peptide-based hydrogel-drug conjugates, illustrated with a selection of representative examples from literature.
Peptide hydrogel-drug conjugates for tailored disease treatment - ScienceDirect
