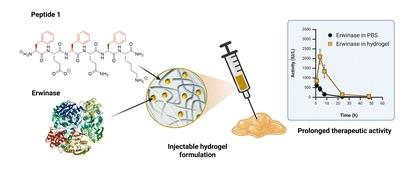
In this study, hexamer peptide-based hydrogels were loaded with different model protein cargos and the release profiles investigated to explore the balance between injectability and loading capacity permitting the release of a therapeutically relevant dose. We demonstrate that the release of protein cargos from our hexamer peptide hydrogels depends on the stability of the hydrogel network, the mobility of the cargo to diffuse out of the network, and the interaction between the hydrogel network and the cargo. For the first time, our peptide hydrogels were used to develop an injectable sustained release formulation of a therapeutic enzyme, namely Erwinase®, an FDA-approved asparaginase for the treatment of acute lymphoblastic leukemia. We show that the current hexamer peptide-based hydrogels allow sufficient protein loading and sustained release of the fully active asparaginase enzyme both in vitro and in vivo. Altogether, this study describes how peptide hydrogels can be exploited to provide injectable slow-release formulations of biologics, including enzyme therapeutics, to enhance their clinical applicability.
Link to Publisher's page:
