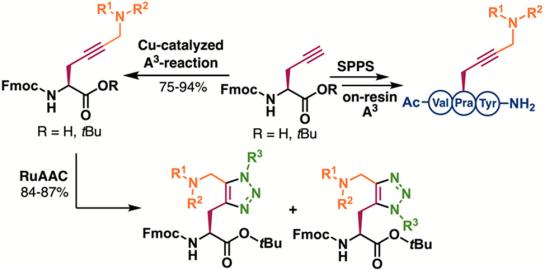
Herein, alkylated propargylamines are reported as constrained lysine mimetics and constructed in a single step using a copper(I)-catalyzed A3-coupling reaction. Using multiple secondary amines, the reaction allowed the generation of a structurally diverse set of N-Fmoc protected amino acid derivatives. In addition, the A3-reaction was applied on solid phase via the assembly of short model tripeptides. Moreover, the internal alkyne moiety allowed further functionalization toward novel 1,4,5-trisubstituted 1,2,3-triazole-based amino acids.
Click here for publisher's page
