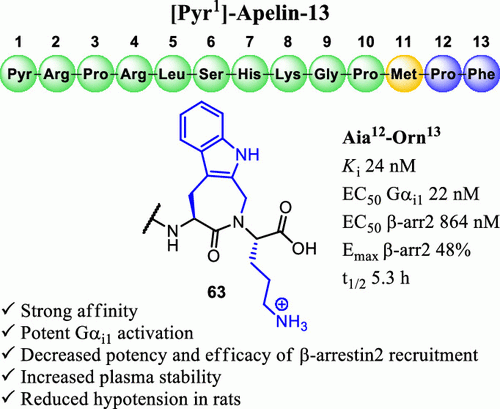
Apelin is an endogenous peptide that is involved in many diseases such as cardiovascular diseases, obesity, and cancer, which has made it an attractive target for drug discovery. Herein, we explore the penultimate and final sequence positions of [Pyr1]-apelin-13 (Ape13) via C-terminal Nα-alkylated amide bonds and the introduction of positive charges, potentially targeting the allosteric sodium pocket, by assessing the binding affinity and signaling profiles at the apelin receptor (APJ). Synthetic analogues modified within this segment of Ape13 showed high affinity (Ki 0.12–0.17 nM vs Ape13 Ki 0.7 nM), potent Gαi1 activation (EC50 Gαi1 0.4–0.9 nM vs Ape13 EC50 1.1 nM), partial agonist behavior disfavoring β-arrestin 2 recruitment for positively charged ligands (e.g., 49 (SBL-AP-058), EC50 β-arr2 275 nM, Emax 54%) and high plasma stability for N-alkyl ligands (t1/2 > 7 h vs Ape13 t1/2 0.5 h). Combining the benefits of the Nα-alkylated amide bond with the guanidino substitution in a constrained ligand led to 63 (SBL-AP-049), which displayed increased plasma stability (t1/2 5.3 h) and strong reduction of β-arrestin 2 signaling with partial maximal efficacy (EC50 β-arr 864 nM, Emax 48%), significantly reducing the hypotensive effect in vivo.
