Molecular design and synthesis
Through various synthetic methods featuring, in some cases, stereoselective transition-metal and enzyme-catalyzed processes, as well as multiple component reactions using iminium ion cyclizations, ORGC has created a wide variety of heterocyclic structures that serve as privileged templates for medchem purposes or can be considered as building blocks to be integrated in more complex biomolecules, such as peptides. Notably, the 4-amino-arylazepinone scaffolds developed by ORGC have been broadly applied to create valuable ligands of the opioid, melanocortin, somatostatin, and neurotensin receptors. These gave rise to ligands that are much more druggable, as compared to the native, natural ligands. As such, ORGC is using the prepared heterocycles to develop GPCR ligands or transport vectors able to cross the blood-brain barrier and other physiological membranes. The group has, for example, recently disclosed novel cell-penetrating oligomers that outperform the established peptides.
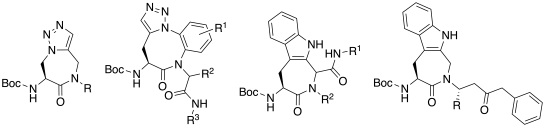
The main research lines are exemplified below:
"Amino Triazolo Diazepines (Ata) as Constrained Histidine Mimics."
Buysse, K.; Farard, J.; Nikolaou, A.; Vanderheyden, P.; Vauquelin, G.; Pedersen, D. S.; Tourwe, D.; Ballet, S., Org. Lett. 2011, 13, 6468-6471.
"Highly Diastereoselective Synthesis of 1-Carbamoy1-4-aminoindoloazepinone Derivatives via the Ugi Reaction."
Jida, M.; Betti, C.; Urbanczyk-Lipkowska, Z.; Tourwe, D.; Ballet, S., Org. Lett. 2013, 15, 5866-5869.
"Efficient synthesis of conformationally constrained, amino-triazoloazepinone-containing di- and tripeptides via a one-pot Ugi-Huisgen tandem reaction."
Barlow, T. M. A.; Jida, M.; Tourwe, D.; Ballet, S., Org. Biomol. Chem. 2014, 12, 6986-6989.
"Efficient one-pot synthesis of amino-benzotriazolodiazocinone scaffolds via catalyst-free tandem Ugi-Huisgen reactions."
Barlow, T. M. A.; Jida, M.; Guillemyn, A. K.; Tourwe, D.; Caveliers, V.; Ballet, S., Org. Biomol. Chem. 2016, 14, 4669-4677.
"Indoloazepinone-Constrained Oligomers as Cell-Penetrating and Blood-Brain-Barrier-Permeating Compounds."
Van der Poorten, O.; Legrand, B.; Vezenkov, L. L.; Garcia-Pindado, J.; Bettache, N.; Knuhtsen, A.; Pedersen, D. S.; Sanchez-Navarro, M.; Martinez, J.; Teixido, M.; Garcia, M.; Tourwe, D.; Amblard, M.; Ballet, S, ChemBioChem 2018, 19, 696-705.
Alkyl quinolones (orange box) such as 4-hydroxy-2-heptylquinoline (HHQ) and their respective “N-Oxides” (blue box) are common secondary metabolites occurring in plants as well as bacteria. They can be involved in bacterial signalling (“quorum sensing” in Pseudomonas aeruginosa) and often show significant antibiotic properties. The Hennecke group / ORGC is preparing various new derivatives of these substances for collaborative research with microbiology (group of Prof. Dr. Susanne Fetzner, University of Münster) and medicinal chemistry.
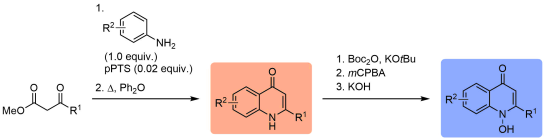
See for example:
“Synthesis and biological activity of methylated derivatives of the Pseudomonas metabolites HHQ, HQNO and PQS”
S. Thierbach, M. Wienhold, S. Fetzner, U. Hennecke, Beilstein J. Org. Chem. 2019, 15, 187-193.
“Chemical Modification and Detoxification of the Pseudomonas aeruginosa Toxin 2-Heptyl-4-hydroxyquinoline N-Oxide by Environmental and Pathogenic Bacteria”
S. Thierbach, F. S. Birmes, M. C. Letzel, U. Hennecke, S. Fetzner, ACS Chem. Biol. 2017, 12, 2305-2312.
