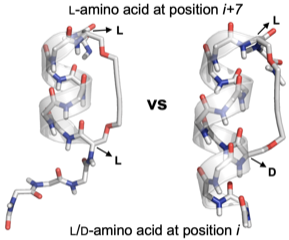
Short alphahelical peptide sequences were stabilized through Glaser‐Hay couplings of propargylated L‐ and/or D‐serine residues at positions i and i+7. NMR analysis confirmed a full stabilization of the helical structure when a D‐Ser (i), L‐Ser (i+7) combination was applied. In case two l‐Ser residues were involved in the cyclization, the helical conformation is disrupted outside the peptide's macrocycle.
