The growing need for protein-based theranostics calls for precise, efficient conjugation strategies. Here, we report the first application of VyPAL2 — one of the fastest known peptide ligases — for site-specific radiolabeling of single-domain antibodies, offering a powerful enzymatic tool for next-generation nuclear imaging and therapeutic development.
An anti-hPDL1 sdAb carrying a C-terminal NQL-tag was modified using two strategies: (1) “Prep & Pair”, combining VyPAL2 with human glutaminyl cyclase (hQPCT) to ligate a methyl-tetrazine handle for subsequent click-radiofluorination with Al[18F]F-NODAGA-BCN, and (2) “Fuse & Use”, to install a NOTA chelator for immediate 68Ga-labeling.
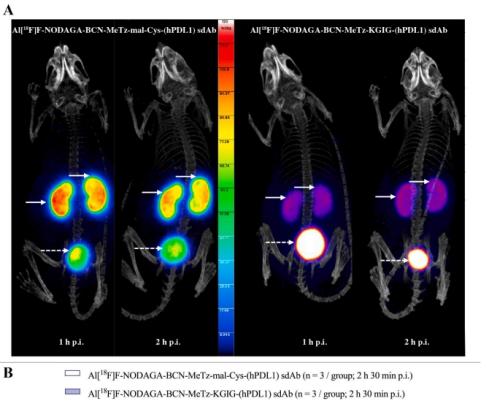
Both strategies produced functionalized sdAbs within 15 min at 25 °C, yielding 55–65 % of recovered product with >99 % purity. Radiolabeled sdAbs demonstrated high radiochemical purity (> 95 %) and apparent molar activities up to 37 GBq/μmol, with preserved target affinity (KD 3–4 nM). In vivo at 2 h 30 p.i., enzymatically modified tracers exhibited significantly reduced renal retention compared to conventional maleimide-cysteine conjugates (35.5 ± 5.7 vs. 105.5 ± 10.1 %IA/g, p < 0.001). The [68Ga]Ga-NOTA-sdAb accumulated specifically in hPDL1POS xenografted tumors (2.77 ± 0.32 %IA/g), achieving high tumor-to-blood (19.0 ± 1.9) and tumor-to-muscle (51.3 ± 15.7) ratios.
VyPAL2-mediated ligation thus represents a rapid, robust strategy for sdAb functionalization, supporting the development of next-generation protein-based radiopharmaceuticals.
Link to Publisher’s page: https://doi.org/10.1016/j.jconrel.2025.114361
Link to Publisher’s page: https://doi.org/10.1016/j.jconrel.2025.114361
