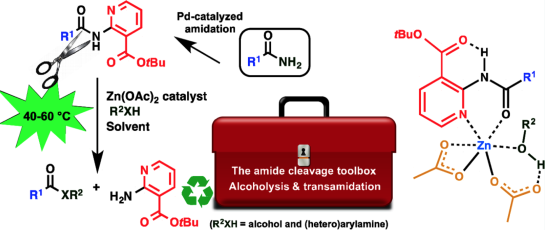
A two-step catalytic amide-to-ester transformation of primary amides under mild reaction conditions has been developed. A tert-butyl nicotinate (tBu nic) directing group is easily introduced onto primary amides via Pd-catalyzed amidation with tert-butyl 2-chloronicotinate. A weak base (Cs2CO3 or K2CO3) at 40–50 °C can be used provided that 1,1′-bis(dicyclohexylphosphino)ferrocene is selected as ligand. The tBu nic activated amides subsequently allow Zn(OAc)2-catalyzed nonsolvolytic alcoholysis in tBuOAc at 40–60 °C under neutral reaction conditions. The activation mechanism is biomimetic: the C3-ester substituent of the pyridine in the directing group populates the trans-conformer suitable for Zn-chelation, C═Oamide–Zn–Ndirecting group, and Zn-coordinated alcohol is additionally activated as a nucleophile by hydrogen bonding with the acetate ligand of the catalyst. Additionally, the acetate ligand assists in intramolecular O-to-N proton transfer. The chemoselectivity versus other functional groups and compatibility with challenging reaction partners, such as peptides, sugars, and sterols, illustrates the synthetic applicability of this two-step amide cleavage method. The tBu nic amides do not require purification before cleavage. Preliminary experiments also indicate that other weak nucleophiles can be used such as (hetero)arylamines (transamidation) as exemplified by 8-aminoquinoline.
